BioXmark® shows promising clinical results for intraoperative marking of tumor resection surface to guide postoperative radiotherapy of oral cancer
17 December 2021
- Intraoperative injection of several small BioXmark® markers was found to be a safe and feasible approach to effectively mark the oral tumor resection surface for postoperative radiotherapy planning
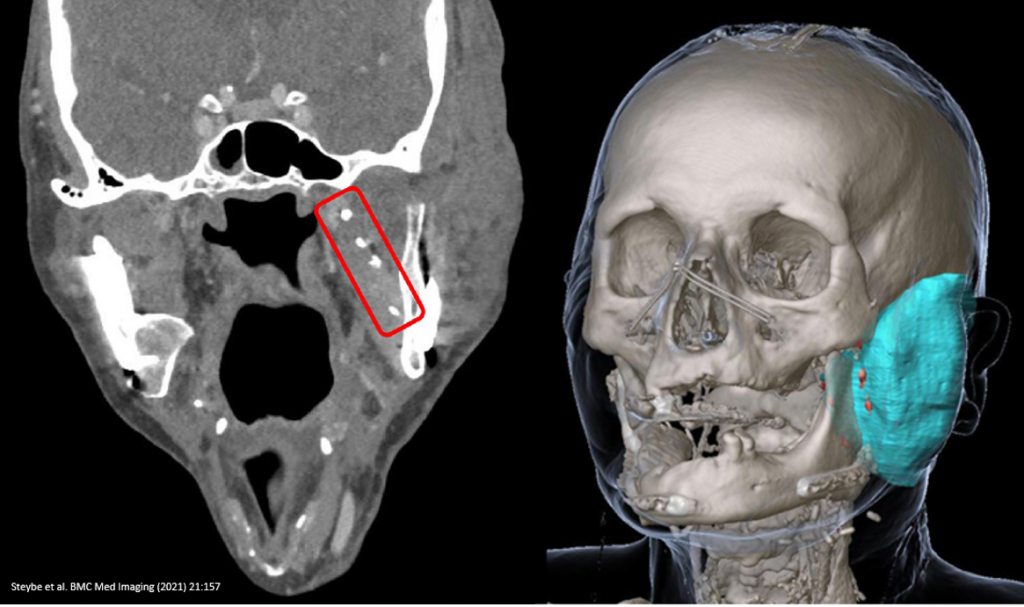
- BioXmark® markers as small as 10 μl were found to be visible on CT
DK-Copenhagen, December 17, 2021 – Nanovi informs that Dr. David Steybe and colleagues from the Department of Oral and Maxillofacial Surgery at the University of Freiburg have published their second paper on BioXmark® in head and neck cancer.
BioXmark® is a liquid soft tissue marker, and this second paper presents the results of a clinical study evaluating BioXmark® for intraoperative marking of the tumor resection surface in oral cancer patients planned for postoperative radiation therapy. The results confirmed the previous findings, obtained for BioXmark® in an animal model, in human patients.
Study design
The recent study evaluated BioXmark® ex-vivo and in-vivo. The ex-vivo part was aimed at assessing the CT X-ray settings on visibility and discriminability of low volume injections markers. This part was conducted in an animal model. The subsequent in-vivo study evaluated the transferability of the ex-vivo findings into the clinical setting and assessed the visibility of low volume BioXmark® markers in both single- and dual-energy CT to evaluate the potential of dual-energy CT to discriminate the visualization of BioXmark® markers from calcified and metal structures for optimized delineation guidance.
Results
The ex-vivo part of the study found that it was possible to make CT-based differentiation both between two different marker sizes (10 μl vs. 30 μl) and among the individual markers.
The in-vivo evaluation of BioXmark® in single-energy CT imaging found that “intraoperative injection of 66 SAIB/x-SAIB [BioXmark®] markers in a patient undergoing surgical resection of a squamous cell carcinoma located at the base and lateral margin of the tongue resulted in 57 markers well identifiable as hyperdense structures in postoperative CT imaging”.
The clinical evaluation of the marker in dual-energy CT imaging found: “…43 of 52 marker injections resulted in hyperdense structures well identifiable in the mixed energy images and providing the basis for three-dimensional reconstruction of the tumor resection surface/flap volume” and “…Dual Energy-CT images could be demonstrated to provide useful assistance in delineating SAIB/x-SAIB [BioXmark®] markers from metal clips and calcified structures…”.
Regarding the feasibility of applying BioXmark® intraoperatively, the study found that “Intraoperative creation of markers by injecting SAIB/x- SAIB [BioXmark®] proved to be a straightforward procedure. Evaluating the visibility of the markers in routine postoperative CT imaging revealed 83% and 86% respectively of the SAIB/x-SAIB [BioXmark®] injections to be clearly visible”.
The present study demonstrated BioXmark® markers as low as 10 μl to be clearly visible in CT imaging with a low level of artefacts. This can be considered beneficial in the context of assessing surrounding structures in postoperative and follow-up imaging. 10 μl volume markers injected with low distances among them can facilitate reliable delineation of the tumor resection surface in postoperative imaging, focal injections of 30 μl can be applied to delineate areas of particular interest in postoperative imaging.
Conclusion
In conclusion, the study found that intraoperative injection of low volume BioXmark® markers can be considered a promising option to facilitate the identification of tumor resection surface in postoperative CT imaging for RT planning and follow-up imaging.
References:
Steybe D, Poxleitner P, Voss PJ, Metzger MC, Schmelzeisen R, Bamberg F, Kim S, Russe MF. Evaluation of computed tomography settings in the context of visualization and discrimination of low dose injections of a novel liquid soft tissue fiducial marker in head and neck imaging. BMC Med Imaging. 2021 Oct 27;21(1):157. doi: 10.1186/s12880-021-00689-y. PMID: 34702192; PMCID: PMC8549337.